Abstract
Background Recently, two chimeric antigen receptor T-cell (CAR-T) constructs have been FDA approved for relapsed/refractory multiple myeloma (RRMM) after exposure to at least 4 prior lines of therapy. High tumor burden has been associated with inferior outcomes, reduced CAR-T expansion and higher rates of toxicity. Patients relapsing after multiple lines of therapy more frequently have oligosecretory and extra-medullary disease that may not be well captured by serum-based markers of disease or bone marrow infiltration, respectively. Functional imaging with FDG-PET is well established as a useful tool in patients with myeloma but its prognostic impact in highly refractory patients or those proceeding with CAR-T has not been described. Metabolic tumor volume (MTV) capturing all metabolically active disease can be useful as a more global assessment of disease burden. We sought to evaluate tumor burden as measured by MTV in a cohort of patients presenting for anti-BCMA CAR-T with RRMM.
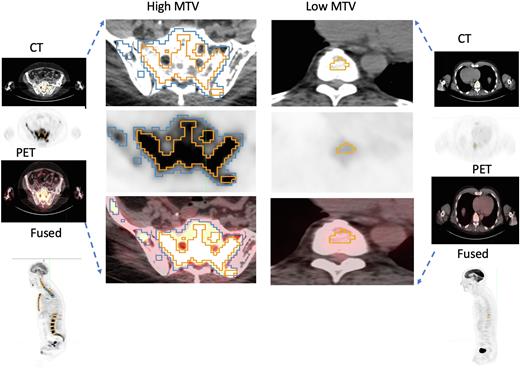
Methods We identified all patients presenting to our center treated with anti-BCMA CAR-T with available restaging imaging performed within 60 days prior to lymphodepleting chemotherapy (LD-chemo). Baseline skull to midthigh with or without leg/whole-body 18F-FDG PET/CT scans were evaluated for MTV using a custom tool implemented on MIM PACS version 7.1 (MIM Software, Cleveland, OH) as previously reported (Dean et al, Blood Adv 2020). Briefly, images were semi-automatically analyzed to identify abnormal regions with reference to average metabolic activity of normal liver defined by PET SUV. Metabolically active volume at the lesion level were converged based on PERCIST criteria (41% of SUVmax). Summation of the metabolically active volumes across the human body is reported as the Metabolically Tumor Volume (MTV), measured in ml. Patient image scans were reviewed centrally by a radiologist blinded to outcomes, who removed any false positive uptake unrelated to metabolically active disease. Patients with the following FISH cytogenetics were considered high risk (HR): t(4;14), t(14;16) and deletion 17p/monosomy 17 whereas the remainder were standard risk (SR). The high and low tumor volume groups were selected based on the median MTV value in cohort. Baseline laboratory tests (e.g. beta2 microglobulin (b2M), C-reactive protein (CRP)) and repeat bone marrow biopsy were obtained prior to LD-chemo. Soluble BCMA (sBCMA) was measured by ELISA on patients with available serum samples (R&D Systems, MN, USA #DY193). All analyses were conducted in Stata (16.1, StataCorp LLC, College Station, TX).
Results The study cohort consisted of 66 patients. Median age was 65 years (range 36 - 81), 55% were male, 55% had ECOG 0-1 prior to LD-chemo and 65% received bridging therapy. 25 (38%) were categorized as HR based on FISH cytogenetics. Patients had received a median of 6 prior lines of therapy and median interval between PET and date of CART infusion was 14.5 days (range 2-54). Median MTV was 26.3ml (0.26-1073.87ml) and only 3/66 (5%) had no measurable PET-avid lesions captured by this algorithm. Patients with high MTV (>26) were more likely to have elevated baseline CRP >0.5mg/dL (p=0.007) and receive bridging therapy (60% vs 40%, p=0.02). MTV correlated with sBCMA levels on day -6 (r=0.82, p=0.003), and with baseline b2M (r=0.47, p=0.005), but MTV did not correlate well with percentage plasma cells in pre-CAR-T bone marrow biopsy (r=0.12, p=0.3). The risk of ³G2 CRS was significantly higher (44% vs 12%, p=0.02) and any grade ICANS was numerically higher (27% vs 12%, p=0.1) in those with high vs low MTV, respectively. Finally, fewer patients with high MTV achieved CR by D30 (19% vs 31%, p=0.2) and with limited follow up 12/14 (86%) of deaths had occurred in patients with high baseline MTV (p=0.003).
Conclusion These preliminary data confirm the added value of MTV calculated from baseline imaging as part of standard of care in patients with heavily pre-treated relapsed myeloma presenting for anti-BCMA CAR-T therapy. MTV correlated highly with sBCMA measured at day-6 and may be more representative for risk assessment than basing burden on bone marrow plasma cell percentage, which has not demonstrated a consistent relationship in predicting adverse events to date. High MTV appears to be associated with an increased risk of CAR-T specific adverse events and inferior outcomes and warrants further investigation.
Disclosures
Hansen:BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria; Survivorship: Honoraria. Baz:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; celgene: Consultancy, Honoraria; karyopharm: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; genentech: Membership on an entity's Board of Directors or advisory committees; Shattuck labs: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria; Merck: Research Funding. Grajales-Cruz:sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Blue:Sanofi: Consultancy, Speakers Bureau; Jassen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria. Shain:Janssen and BMS: Other: PI of clinical trials; AbbVie and Karyopharm: Research Funding; GSK, BMS, Sanofi, Karyopharm, Takeda, Janssen, Adaptive and Amgen: Speakers Bureau; GSK, Janssen and BMS: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb (BMS), Janssen, GlaxoSmithKline (GSK), Adaptive, Sanofi, and Takeda, and Amgen: Honoraria. Alsina:BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Research Funding. Liu:Sanofi: Speakers Bureau. Locke:Society for Immunotherapy of Cancer: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; CAREducation: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; Daiichi Sankyo: Consultancy; Takeda: Consultancy; Sana: Consultancy; CERo Therapeutics: Research Funding; ), National Cancer Institute: Research Funding; Leukemia and Lymphoma Society: Research Funding; Aptitude Health: Other: Education or editorial activity; ASH: Other: Education or editorial activity; BMS: Research Funding; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Freeman:Incyte: Honoraria; Amgen: Honoraria; Sanofi: Honoraria; Janssen: Honoraria, Research Funding; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal